pH Scale
Name: Marilyn Alswede
Date of lesson: Unit Project Week 3 Tuesday
Description of the class:
Length of lesson: 45 – 50 minutes
Source of the lesson:
- Modern Chemistry Texas Edition Publisher Holt, Rinehart, and Winston Chapter 15
- Chemistry: Visualizing Matter Technology Edition Publisher Holt Chapter15
TEKS addressed:
Chemistry (1) Scientific processes. The student, for at least 40% of instructional time, conducts field and laboratory investigations using safe, environmentally appropriate, and ethical practices. The student is expected to:
(A) demonstrate safe practices during field and laboratory investigations
Chemistry (2) Scientific processes. The student uses scientific methods during field and laboratory investigations. The student is expected to:
(B) collect data and make measurements with precision
Chemistry (14) Science concepts. The student knows the properties and behavior of acids and bases. The student is expected to:
(A) analyze and measure common household products using a variety of indicators to classify the products as acids or bases
I. Overview
This lesson introduces students to pH and how to measure the pH of a solution. Students will identify unknown solutions as an acid, a base, or neutral depending upon their measured pH values with both a pH meter and pH indicator paper. As a class, students will discuss the advantages and disadvantages of using a pH meter and pH indicator paper.
II. Performance objectives (learner outcomes)
Students will be able to:
- Compare and contrast pH paper versus pH meter
- Identify if a solution is an acid or a base based upon the measured pH value
- Explain the definition of pH
- Describe the difference of pH values for an acid, a base, or neutrality
III. Resources, materials and supplies (per box/teaching pair)
Per Group:
- pH meter
- graphing calculator
- Lab Pro
- 11 cups
- pH indicator paper strips
- color key for indicator paper
- Squeeze Squirt Bottle
- Student Lab Exercise worksheet
Per Class:
IV. Advanced Preparation:
Calibrate all the pH meters before lab. Prepare ten bottles of the unknown solutions.
VI. Vocabulary & Definitions:
pH- the negative logarithm of the hydrogen ion concentration in a solution
neutral- describes a solution that contains equal concentrations of hydronium ions and hydroxide ions
indicator- dye that changes to different colors in solutions of different pH
VII. Safety Considerations:
Goggles and gloves must be worn during the lab exercise.
Five-E Organization
Teacher Does Probing Questions Student Does
| Engage: Fill a test tube with ammonia. “I have a test tube here filled with ammonia.” “I’m now going to add drop by drop phenolphthalein indicator.” “I’m now going to add vinegar to the test tube.” Time: 5 minutes |
What are some products we use in our everyday life that contain ammonia? What will happen if I add this phenolphthalein indicator? What is phenolphthalein indicator? What will happen if I add vinegar to the test tube? What happened? Why did it turn clear? |
Household cleaners. The solution will turn pink. Phenolphthalein indicator detects when a solution is basic. Noting will happen, or the solution will turn back to clear. Turned clear. The vinegar neutralized the solution to pH of 7. |
| Explore: Today we are going to explore more about acids and bases. We are going to measure the pH of various solutions found in your house, you may be surprised what actually is acidic or basic.“To make this activity more interesting you will have to be like detectives to identify the liquid by measuring the pH and matching the pH value to the given pH values on the pH scale. You will be given 10 different solutions. You also be comparing your measured pH meter value to your measured pH indicator paper value.” Have the students work in groups of 3 students or less. As the students work, assist them with using the pH meters because most likely they’ve never used them before. Also remind students to keep their goggles over their eyes not their forehead. Time: 25 minutes |
What are some pH values that are typical of acids? And Bases? What is the pH of a neutral solution? What is the pH of water? Some questions you might ask depending on how far along the group is: Which solutions have you identified? Is that pH value an acid or a base? |
pH of 1-6 pH of 8-14 pH of 7 7 Students will perform the identification of solutions by measuring pH and completing their worksheet. Students’ answers will vary. Acid or Base |
| Explain: After students have completed the identification of all their solutions, reveal the identity of each solution on the overhead. Do a quick assessment of how many groups correctly identified each solution. “Raise your hand if you identified solution _____ correctly.” Quickly discuss and compare the pH meter and pH indicator paper. Time: 10 minutes |
Were there any solutions that surprised you that you thought were basic but are actually acidic or the other way around? What is the pH meter or pH indicator paper measuring exactly? What are some advantages to using the pH indicator paper? What are some disadvantages of pH indicator paper? What are some advantages of using a pH meter? What are some disadvantages of using a pH meter? |
Milk, apple juice. Students’ answers will vary. It is measuring the concentration of hydrogen ions or protons. pH indicator paper does not cost too much, no calibration needed, it’s a good estimate of the pH, it’s quick and easy. pH indicator paper does not give you an exact pH reading Rapid accurate measurement, continuous recording of pH changes, automatically can graph the change Calibration is required, more expensive, requires a strict storage guideline…must be covered in ionic solution at all times |
| Extend / Elaborate: The human stomach is a very unique organ in our digestive system. The pH of our stomach is 2. Our esophagus lining is not resistant to acid as the lining of our stomach is. The acid causes heartburn pain which we call it because it’s in the general location of our heart. The action of the acid as a result of continued indigestion can permanently damage the tongue, throat, esophagus, and damage our teeth by dissolving the enamel. Time: 5 minutes |
What do you think is the pH of the human stomach? What chemical is found in our stomach acid? What happens when this acid comes up from our stomach and into the esophagus? Over time what will happen to our esophagus? |
Acidic, pH of 2 HCl We experience heartburn. Sore spots, holes will form |
| Evaluate: “As a wrap of today’s class, let’s take a short quiz.” Put up the overhead with questions. Time: 5 minutes |
Students will complete quiz individually. |
Name_______________________
Group Members’ Names______________________
Identifying Unknown Solutions by measuring pH
Directions:
1. Fill your 10 cups with the 10 different unknown solutions. Be sure to label them correctly.
2. Use the pH meter to find the pH of each unknown solution. Record your values in the table below. Before you measure a new solution, rinse off the meter with distilled water with your squeeze squirt bottle over a waste cup.
3. Use the pH indicator paper to find the pH of each unknown solution.
4. Use the pH scale on the next page to determine the identity of your unknown solution.
| Unknown Solutions |
pH Meter Value |
pH Indicator Paper |
Identity |
| 1 |
|||
| 2 |
|||
| 3 |
|||
| 4 |
|||
| 5 |
|||
| 6 |
|||
| 7 |
|||
| 8 |
|||
| 9 |
|||
| 10 |
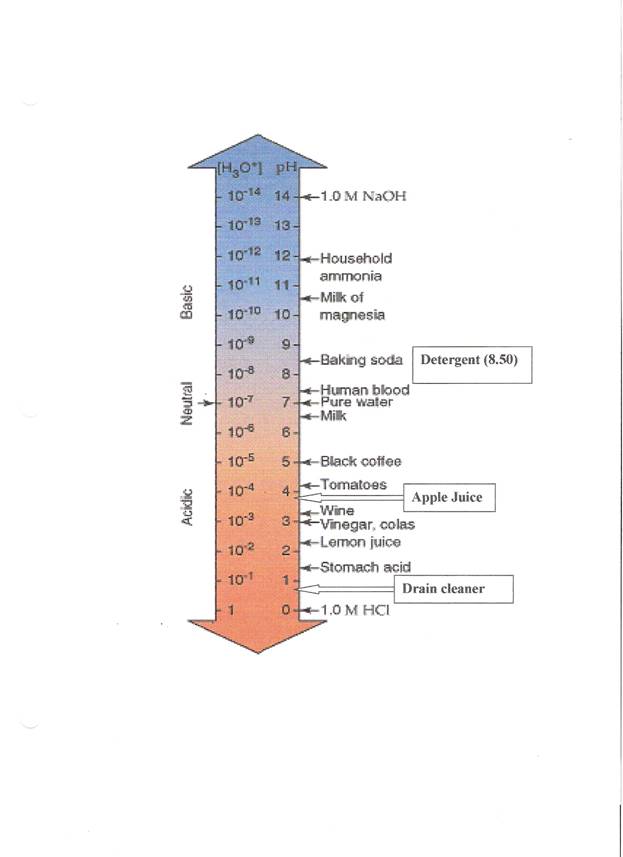



Answer Key
| Unknown Solutions |
pH Meter Value |
pH Indicator Paper |
Identity |
| 1 |
NaOH |
||
| 2 |
Apple Juice |
||
| 3 |
Baking Soda |
||
| 4 |
Cola |
||
| 5 |
Drain Cleaner |
||
| 6 |
Lemon Juice |
||
| 7 |
Detergent |
||
| 8 |
Milk |
||
| 9 |
Vinegar |
||
| 10 |
Water |
pH Quiz
1. If a solution has a pH of 3, is it an acid, a base, or neutral?
2. What is pH a measure of?
3. Compare and contrast a pH meter versus pH indicator paper.
4. Which of the following is true?
a. pH of less than 7 is basic; pH of more than 7 is acidic
b. pH of less than 7 is acidic; pH of more than 7 is basic